Introduction: Thrombopoietin receptor agonists (TPO-RAs) are among the most widely used second-line treatments for immune thrombocytopenia (ITP). TPO-RAs counterbalance platelet destruction by stimulating proliferation and maturation of the megakaryocytes. Response rates varies between 60-90% and in the majority of cases continuous treatment with TPO-RAs is required to maintain response. However, it has recently been shown that 10-30% of patients can maintain response after treatment discontinuation (sustained response off-therapy - SROT). Romiplostim, Eltrombopag, and Avatrombopag are 3 TPO-RAs licensed for use in ITP in many countries. However, there is a lack of comparative studies on the efficacy and safety of these agent.
Aims: To describe real-world use, effectiveness (rates of initial response, durable response, discontinuation and SROT) and safety of TPO-RAs in Norway for each of the 3 agents.
Methods: This is a multicenter, retrospective/prospective study based on data from Norwegian ITP Registry (NOR-ITP). We identified all patients with primary ITP who had received at least one TPO-RA during the course of their disease (n=87). These patients were diagnosed with ITP between 1966 and 2023 and were included in NOR-ITP between 2016 and May 2023. We defined response to treatment as platelet count > 30*10 9/L and double than baseline and absence of bleeding, and complete response as platelet count > 100*10 9/L; time to response as the time to obtain response; durable response as 2 consecutive platelet counts > 30*10 9/L at 6 months without other therapies for ITP; SROT as response for at least 6 months in absence of any therapy for ITP after the discontinuation of TPO-RA. We reported adverse events as registered in NOR-ITP. We stratified the first TPO-RA agents by year ranges (2009 to 2019, 2019 to 2022, and 2022-2023) to evaluate tendency in choice of first agent and treatment line in which first TPO-RA was used.
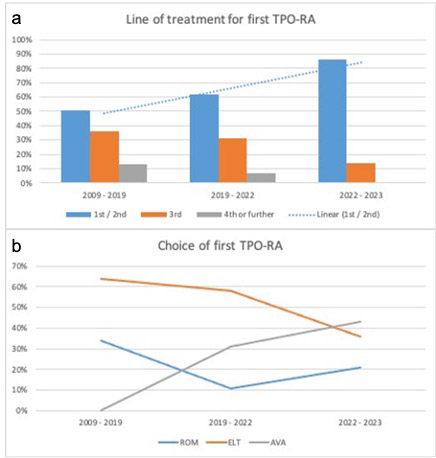
Results: Median (Q1; Q3) age of 87 ITP patients at the time of first TPO-RA was 60 years (38; 68); M:F ratio = 1:1. Phase of ITP in which the first TPO-RA was initiated was newly diagnosed in 14 (16%), persistent in 15 (17%), and chronic in 58 (67%) patients. As first TPO-RA, 23 (26%) received Romiplostim, 50 (57%) Eltrombopag, and 14 (17%) Avatrombopag. As shown in Figure1a, TPO-RAs are increasingly used in in the first and second line in ITP. Eltrombopag was the most widely used agent during 2009-2019, but the use declined from 2019-2022, while the use of Avatrombopag increased to become the mostly used agent in 2022-2023 (Figure1b). Response to first TPO-RA was achieved in 70% (16) for Romiplostim, 68% (35) for Eltrombopag, and 100% (14) for Avatrombopag. A complete response was achieved by 61% (14) for Romiplostim, 56% (28) for Eltrombopag, and 100% (14) for Avatrombopag. Median (Q1; Q3) time to response in days from start of TPO-RA was 7 (5;12) for Romiplostim, 10 (7;14) for Eltrombopag, and 7 (4;14) for Avatrombopag. Durable response rate (at 6 months) was 52% (n=12) for Romiplostim, 74% (n=37) for Eltrombopag, and 64% (n=9) for Avatrombopag. Reasons for discontinuation of Romiplostim were remission in 7 (30%) cases, absence or loss of response in 9 (40%), and was ongoing or discontinued for other reasons in 7 (30%); Eltrombopag was discontinued because of remission in 18 (36%) cases, absence or loss of response in 9 (18%), and was ongoing or discontinued for other reasons in 23 (46%). Avatrombopag was discontinued for remission in 4 (29%) cases, absence or loss of response in 3 (21%), and was ongoing or discontinued for other reasons in 7 (50%). SROT was obtained in 2 (9%) cases for Romiplostim, and 3 (6%) for Eltrombopag. Median duration of SROT was 13 (13; 44) months. Side effects were reported in 22 (25%) patients, 4 fatigue (5%), 2 bone marrow fibrosis (2%), and 2 thrombosis (2%).
Conclusion: Our results show a comparable effectiveness between the three agents, however, a pattern of use of first TPO-RA is changing, and it appears to be slowly turning towards Avatrombopag. This trend is supported by current recommendations from Norwegian medicine funding authority towards Avatrombopag as first choice. Moreover, trust of clinicians towards TPO-RAs is increasing in recent years, as these agents begin to be chosen in earlier lines. Safety profiles were excellent, thrombosis and bone marrow fibrosis were rarely found.
Disclosures
Garabet:Grifols: Honoraria. Pettersen:Sanofi: Honoraria. Tjønnfjord:Novartis: Honoraria; Sobi: Honoraria; GRIFOLS: Honoraria. Tsykunova:Sobi: Honoraria; GRIFOLS: Honoraria. Tran:Novartis: Honoraria; Sobi: Honoraria; GRIFOLS: Honoraria. Ghanima:Argenx: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Sobi, Pfizer: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria; hibio: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Kedrion: Consultancy; cellphire: Consultancy, Honoraria; alpine: Consultancy, Honoraria; BMS: Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal